
A D I V I S I O N O F E K L A V Y A E D U C A T I O N F O U N D A T I O N
| Code: TLM- Sc- 001 | Age: 10-13 yrs | Name: Valency Cards (Chemistry) |
Valency
Cards (Click to view Video)
Valency
is the most fundamental concept in chemical bonding, chemical reaction
etc. This game helps children learn the concept of valency and its
application. Children combine various valency cards and learn the
formula of compound. It helps children develop a mental picture of the
various ions. This experience helps them to say the formula when two
ions are given without actually playing the game.
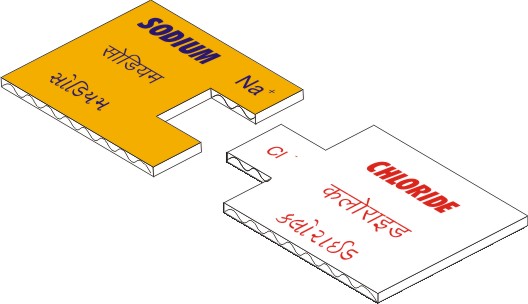
Each set contains
Jigsaw shaped pieces representing positive
and negative valencies. The pieces are colour coded
(positives are yellow and negatives are red). The ions
also have the names written in English, Gujarati &
Hindi. For e.g.
| How to use |
| This material can be used by a single student or two
students. It helps them visualise the concept of Valency.
The Chemical Formulae of common chemical compounds can
also be determined by using the material as described
below. Step 1: Group of 2 children is formed. The Valency Cards are given to them.. Step 2: Each student takes a fresh sheet of paper, draws a margin, writes her name and date on the top right hand corner and name of the exercise in the center of the page. Step 3: Then they create a table as shown below.
|
|||||||||||||||||||||
| The educator reads out
the names of the chemical compounds to them. Each student
determines the Chemical Formula using this material. Step 4: The educator need not give the compounds described below which are only for illustration. She can choose any 10 compounds as long as they fit into this material.
|
|||||||||||||||||||||
| Step 5: For
determining the Chemical Formula all the pieces are
arranged - Yellows (positive ions) on the left and Reds
(negative ions) on the right as shown below:
3. Then the player
picks up the other ion card, Chloride or Cl- and places
it against the root ion 3. Then the players pick up the other ion card, Chloride or Cl- and place it against the root ion. Immediately the players realise that the picture is incomplete and that one more piece is required to complete the picture. Then the players counts the number of pieces of each ion i.e. 1 Ca (Calcium) and 2 Cl (Chloride) gives the compound CaCl2. Step 7: To check, the educator sees the formula and also the various pieces fitted together to confirm answers. Development aspects: 1. Valency is one of
the most fundamental concepts in chemical bonding,
chemical reactions, etc. This game is used to help
children learn the concept of valency and its application
in Chemistry. a) CuSO4 + Mg MgSO4 + Cu b) ZnSO4 + MgCl2 MgSO4 + MgCl2 Mg displaces Zn in Zinc
Sulphate Solution |